Traceability gaps in MedTech?
Protecting the Invisible Links: A New Standard in Critical Parameter Traceability
INTELLIGENT MANUFACTURING TRANSFORMATIONRISK MANAGEMENT
Manfred Maiers
4/29/20253 min read


The Problem: Critical but Untraceable
In highly regulated industries such as medical device manufacturing, aerospace, and automotive, product safety and performance hinge on a network of critical parameters, dimensions, and process steps. These elements often originate from:
Stakeholder requirements (e.g., clinical needs, customer specifications)
Design and Process FMEAs (as mitigations to high-risk failure modes)
Regulatory and compliance standards
Despite their criticality, these elements lose traceability as they flow through the product lifecycle. For example:
A diameter tolerance in an infusion pump designed to prevent over-infusion may appear on a drawing—but the source (a DFMEA mitigation for patient safety) is nowhere documented.
A critical process step to avoid contamination might be captured in a work instruction—but lacks reference to its origin in the PFMEA or control plan.
A material or color specification may be dictated by a customer requirement but gets disconnected from the originating contract or requirement database.
Over time, these vital links become invisible. And when cost reductions, design updates, or manufacturing changes are implemented, critical parameters can be unknowingly modified or removed—reintroducing risks that were previously mitigated.
The Consequences: Hidden Risks, Real-World Impacts
The lack of traceability has real, often severe consequences:
Design drift: Over multiple revisions, the rationale for tolerances or process controls is lost.
Noncompliance: Auditors and regulatory bodies (FDA, ISO) expect traceability from requirement to execution.
Product failure: An “optimized” component or process may reintroduce a previously mitigated failure mode.
Reputation and liability: In industries like MedTech, these oversights can lead to recalls, lawsuits, or worse—patient harm.
The Solution: Introducing the CP²T Tag
To combat this, we developed the CP²T Tag: a bidirectional traceability system for Critical Parameters, Critical Dimensions, and Critical Processes. It links source documents (e.g., stakeholder requirements, DFMEAs, PFMEAs) to downstream outputs (drawings, work instructions, control plans) and back again.
🔁 Bidirectional by Design
Each tag provides:
A forward trace from the source to the point of implementation
A backward trace from the implementation (e.g., a drawing or SOP) to the source rationale
This means that anyone reviewing a document—a quality engineer, a manufacturing tech, a design lead—can instantly understand:
Why a dimension exists
What failure mode it's protecting against
Where else it shows up across documents
What’s at stake if it's changed
CP²T Tag Format Example
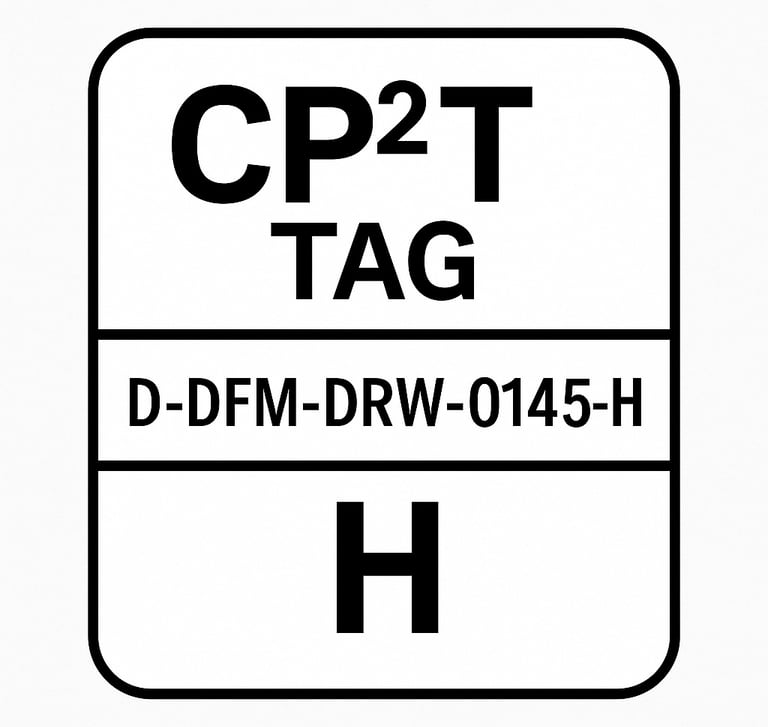
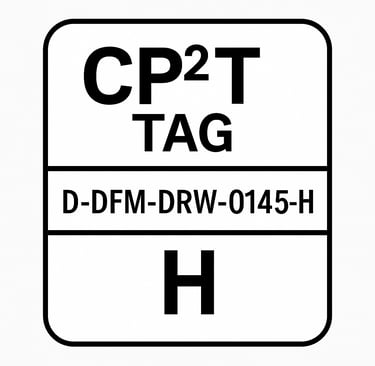
CP2T-D-DFM-DRW-0145-H
Breakdown:
D: Critical Dimension
DFM: Originated in DFMEA
DRW: Implemented in a Drawing
0145: Unique identifier
H: High risk (or RPN value)
This tag would appear next to the dimension on the drawing and be cross-referenced back to the DFMEA row that justified it.
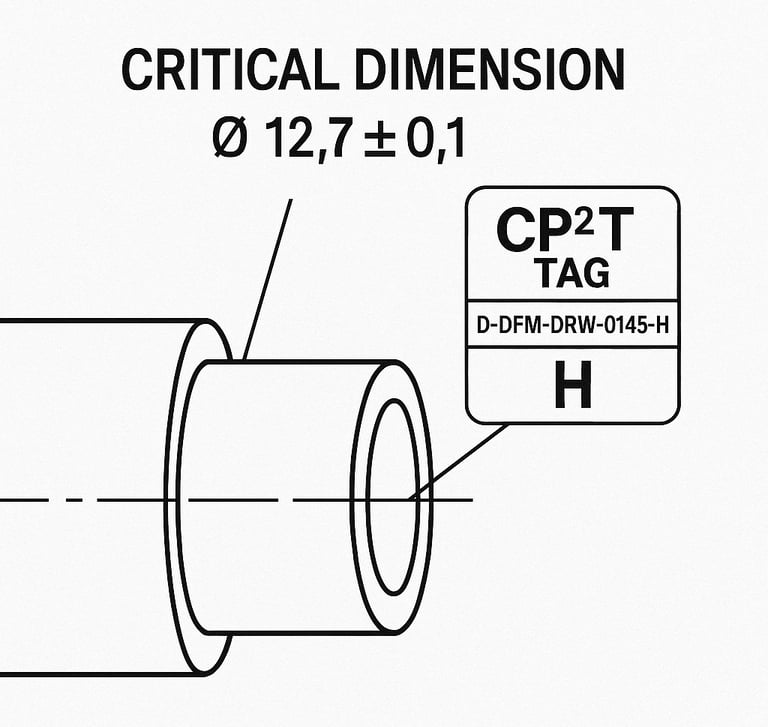
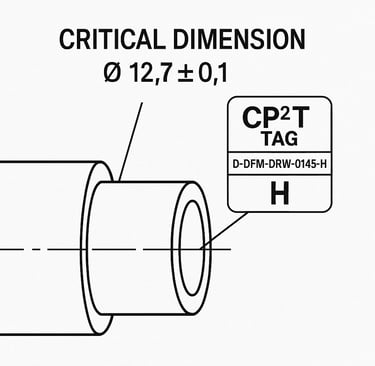
Why CP²T Tags Are a Game-Changer for MedTech
✅ Improved Audit Readiness
CP²T Tags create clear evidence of traceability—making regulatory audits smoother, faster, and more robust.
✅ Built-in Risk Awareness
Each tag surfaces the risk classification or RPN so that teams immediately know which dimensions or steps matter most.
✅ Design Change Control
When a drawing or work instruction is revised, engineers will immediately see which elements should not be altered without reevaluating the original risk.
✅ Enhanced Cross-Functional Communication
Quality, R&D, and manufacturing often operate in silos. CP²T Tags create a shared language around what’s critical and why.
✅ Digital-Ready and Scalable
Tags can be incorporated into PLM systems, document control tools, and barcode/QR systems, enabling real-time linking and status updates.
From Compliance to Confidence
Traceability isn’t just a regulatory checkbox. It’s a foundation of product safety and reliability—especially in industries where lives are on the line.
The CP²T Tag system brings visibility, structure, and accountability to the elements that matter most. It closes the loop between intention and execution, helping teams protect the decisions they made to ensure safety, quality, and compliance.
It’s time to stop losing sight of the critical. It’s time to make traceability a two-way street. It’s time for CP²T Tags.
