FMEA 4.0 Re-Thinking DFMEA Organization (ARTICLE 3)
From Process Functions to System Use Cases & Failure.
THE LEARNING LOOPINTELLIGENT MANUFACTURING TRANSFORMATIONRISK MANAGEMENT
Manfred Maiers
1/15/20263 min read


Re-Thinking DFMEA Organization
From Process Functions to Use Cases & Event Trees
Abstract
Design Failure Modes and Effects Analysis (DFMEA) is a cornerstone of medical device risk management. Yet many DFMEAs remain organized around process functions—a legacy structure that obscures system intent, weakens traceability, and collapses under product complexity.
This article presents a regulator-credible, systems-engineering-aligned alternative: organizing DFMEA around Intended Use → Use Cases → Event Tree Analysis (ETA), then assigning DFMEA attributes to each path.
The result is a DFMEA that is complete, scalable, auditable, and AI-ready.
1. The Legacy Model: DFMEA Organized by Process Function
How It Typically Looks
In the traditional approach, DFMEA rows are grouped by “process functions” such as Power On, Deliver Therapy, Monitor Sensor, or User Interface. Each function becomes a container for potential failure modes, effects, causes, and controls.
Why This Model Persists
Familiarity and inertia
Ease of starting from a requirements list
Spreadsheet convenience (one tab, one list)
Unfortunately, these conveniences mask structural weaknesses that grow with device complexity.
2. The Hidden (and Not-So-Hidden) Problems
Below is an expanded and systematic critique of the process-function-based DFMEA.
2.1 Incomplete Coverage Is Hard to Detect
No objective way to confirm all relevant functions have been captured
Functions are often derived informally, not from a formal system model
Missing functions remain invisible until late design reviews—or worse, post-market
2.2 No Natural Ordering or Hierarchy
Functions appear as a flat list with no system context
No distinction between primary, secondary, or conditional behavior
Cross-cutting functions (e.g., alarms, data handling) get duplicated or fragmented
2.3 No Traceability to Intended Use or Use Environment
Functions rarely map cleanly to Intended Use statements
Use environments (home, clinical, emergency) are implicit or ignored
Reasonably foreseeable misuse is difficult to integrate coherently
2.4 Weak Alignment with System Architecture
Hardware, software, and human interaction blur together
Subsystem boundaries are unclear
Integration failures are discovered late because the DFMEA is not system-aware
2.5 DFMEAs Become Unmanageable
Large devices produce hundreds or thousands of rows
Navigation, filtering, and review become error-prone
Excel-based DFMEAs become brittle, slow, and audit-unfriendly
2.6 Redundancy and Inconsistency
Same failure mode appears under multiple functions
Severity and occurrence rankings drift over time
Control effectiveness is assessed inconsistently
2.7 Poor Support for Top-Down Risk Analysis
Process functions encourage bottom-up thinking only
Failure propagation paths are not visible
System-level hazards are back-filled rather than derived
2.8 Lifecycle and Change Management Failures
Design changes require manual scavenger hunts
Variant and accessory impacts are hard to assess
Post-market data does not map cleanly back to functions
2.9 Audit and Regulatory Risk
Traceability is implicit, not explicit
Justifying completeness during audits is difficult
Reviewers must infer logic rather than verify structure
2.10 Not AI- or Database-Ready
Flat spreadsheets resist relational modeling
No semantic structure for automated reasoning
Limits future digital transformation
3. A Superior Model: Use Cases + Event Tree Analysis
To fix DFMEA, we must change the organizing principle.
Instead of asking:
“What functions does the design perform?”
We ask:
“How is the system intended to be used, and what can go wrong along each usage path?”
This aligns DFMEA with systems engineering and risk management best practices as expected under International Organization for Standardization risk frameworks.
4. Step 1 — Start with Intended Use & Use Cases
Intended Use as the Anchor
Every DFMEA must be traceable to:
Intended medical purpose
Target population
Use environment
User profile
Deriving Use Cases
Use Cases describe real operational scenarios, such as:
Normal therapy delivery
Setup and configuration
Interrupted use
Maintenance and cleaning
Foreseeable misuse
Each Use Case becomes a first-class DFMEA container, replacing “process functions.”
Benefits
Forces completeness
Makes assumptions explicit
Naturally includes user interaction and environment
5. Step 2 — Apply Event Tree Analysis (ETA)
Why Event Trees?
Event Tree Analysis models what happens after an initiating event, capturing:
Normal progression
Deviations
Detection points
Failure propagation
ETA in DFMEA
For each Use Case:
Identify initiating events
Map success and failure branches
Capture conditional paths (e.g., alarm works vs. alarm fails)
Identify end states (safe, degraded, hazardous)
This creates a top-down, causal structure that process functions cannot provide.
6. Step 3 — Assign DFMEA Attributes to Each Path
Instead of one monolithic table, DFMEA attributes are contextually assigned.
For each Use Case × Event Tree Path:
System and subsystem requirements
Functional intent
Potential failure modes
Failure causes
Local, system, and user/patient effects
Existing design controls
Detection and prevention mechanisms
Risk estimation parameters
Risk control measures
Verification and validation activities
This preserves DFMEA rigor while dramatically improving clarity.
7. Structural & Practical Advantages
Completeness
Coverage is proven by Use Case enumeration
Gaps are visible immediately
Traceability
Intended Use → Use Case → Event Tree → DFMEA
Clean linkage to hazard analysis and risk controls
Scalability
Large systems decompose naturally
Variants and accessories map to specific Use Cases
Lifecycle Maintainability
Design changes impact specific paths
Post-market signals map cleanly back to scenarios
Audit Readiness
Logical, defensible structure
Reviewers can follow reasoning without inference
AI & Digital Readiness
Natural fit for relational databases
Enables future AI-assisted risk reasoning
Supports knowledge graphs and structured analytics
8. Comparison: Old vs. New
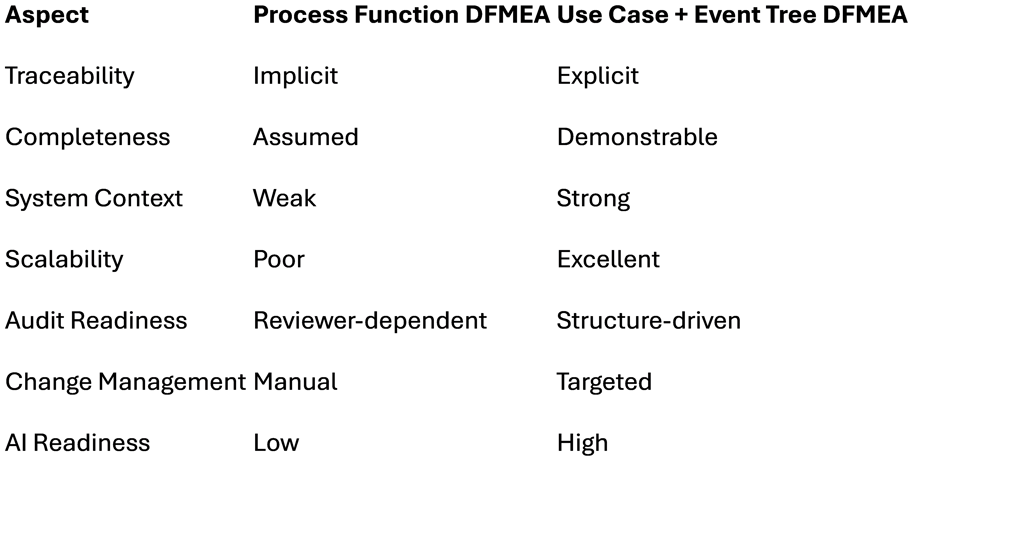

9. Common Objections — and Why They Fail
“This is too complex.”
Complexity already exists—it is merely hidden. This model reveals it early.
“Regulators expect traditional DFMEAs.”
Regulators expect effective risk management, not legacy formatting.
“Our tools don’t support this.”
That is a tooling limitation, not a risk-management justification.
10. Conclusion
Organizing DFMEA by process function is no longer sufficient for modern medical devices. A Use Case– and Event Tree–based DFMEA restores system intent, strengthens traceability, and aligns DFMEA with how regulators, engineers, and—soon—AI systems reason about risk.
This is not DFMEA 2.0.
It is DFMEA done right.
