Enhancing Value Stream Mapping with Quality Data: A Game Changer for MedTech Manufacturing (Part 1)
In Medical Device Manufacturing, compliance, quality, and efficiency are inseparable. Lean tools such as Value Stream Mapping (VSM) have long been used to visualize the flow of materials and information across production processes, helping organizations find waste and improve efficiency. Yet, there is a critical gap in the traditional approach: quality data is missing from the map.
INTELLIGENT MANUFACTURING TRANSFORMATIONTHE LEARNING LOOP
Manfred Maiers
11/10/20252 min read

Enhancing Value Stream Mapping with Quality Data: A Game Changer for MedTech Manufacturing
In Medical Device Manufacturing, compliance, quality, and efficiency are inseparable. Lean tools such as Value Stream Mapping (VSM) have long been used to visualize the flow of materials and information across production processes, helping organizations find waste and improve efficiency.
Yet, there is a critical gap in the traditional approach: quality data is missing from the map.
Most process step boxes in a VSM have efficiency-related attributes; cycle time, changeover time, takt time, and lead time. These are essential, but they do not reflect the true performance of the system. Defects, rework, and scrap rates are rarely included, leaving a blind spot between what looks efficient on paper and what reaches the patient safely and compliantly.
From VSM to EVSM – The Next Step
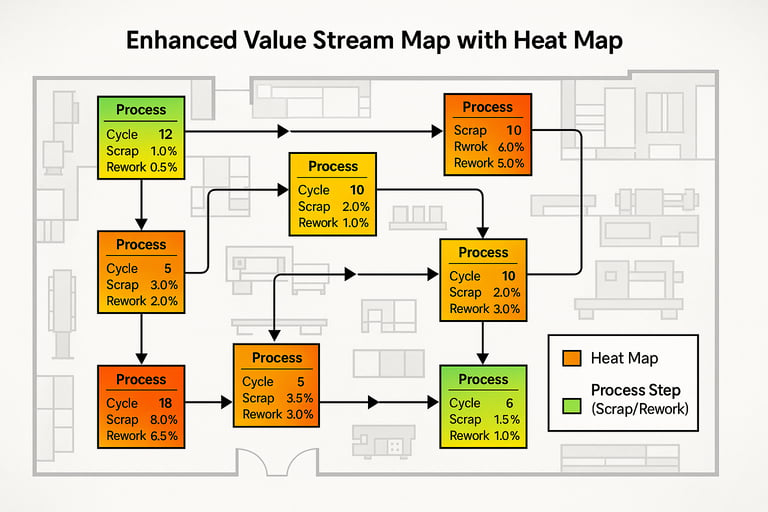
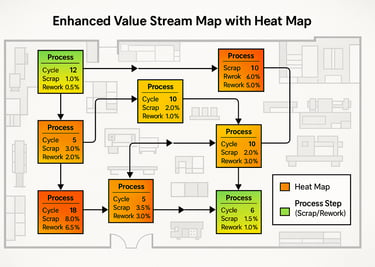
By integrating quality attributes (such as defect rates, rework, and scrap) into the process boxes, the Value Stream Map transforms into an Enhanced Value Stream Map (EVSM).
This enhancement provides several breakthroughs:
First Pass Yield (FPY) & First Time Yield (FTY): Instead of simply mapping the efficiency of a step, we can now measure the true yield of both individual processes and the entire value stream.
Visual Heat Maps: Overlaying the EVSM on the physical plant layout creates a real-time, intuitive heat map of where scrap, rework, or inspection failures concentrate. High-risk areas become visually obvious.
Cross-Stream Insights: Shared process cells or machines across different value streams can be compared and overlaid, highlighting systemic weaknesses versus isolated issues.
Linking to PFMEA and Risk Management
The real power comes when these quality metrics feed directly into the Process FMEA (PFMEA):
Each process step box can be linked to PFMEA failure modes.
Scrap or rework data gives real-world input into Occurrence ratings.
Inspection failures highlight weaknesses in Detection controls.
This creates a closed-loop risk management system where production reality continuously informs the risk profile.
In effect, the EVSM bridges Lean efficiency tools with regulatory risk management tools, something that has historically been siloed.
Why This Matters for MedTech Manufacturing
1. Regulatory Compliance
Regulators expect objective evidence that processes are controlled, and risks are mitigated. An EVSM provides a transparent, data-rich view of the process, linked directly to PFMEA. This strengthens compliance with FDA 21 CFR 820, ISO 13485, and EU MDR requirements for risk-based quality management.
2. Quality Assurance
Instead of reacting to CAPAs, EVSM allows proactive monitoring of where failure modes are most likely to occur. This supports better control plans, more effective verification strategies, and prevention of repeat issues.
3. Operational Excellence
Heat maps of inefficiency and quality losses provide immediate targets for Kaizen events, resource allocation, and automation investments. FPY/FTY analysis across the full value stream allows leadership to prioritize improvements that have the biggest impact on throughput and cost.
A Strategic Advantage
Medical device manufacturing is under increasing pressure: more complex products, tighter regulations, and higher cost expectations. The Enhanced Value Stream Map is not just an incremental improvement, it is a strategic bridge between compliance, quality, and operational excellence.
By embedding rework, scrap, and inspection data into the map, manufacturers unlock a new way to:
Drive continuous improvement.
Support data-driven regulatory audits.
Improve patient safety by addressing process risks early.
Improve plant performance with precision targeting.
In short: EVSM transforms Value Stream Mapping from a Lean visualization tool into a holistic quality and compliance engine.
